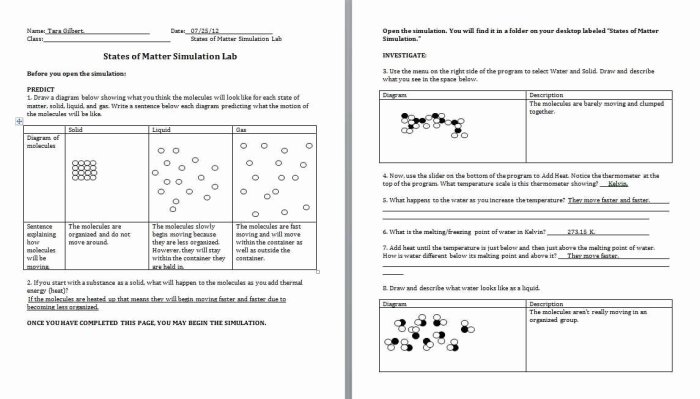
Find the ph of a 0.010mhno2 solution. – Delving into the intriguing realm of chemistry, we embark on a journey to unravel the mysteries of pH and its profound impact on our world. Beginning with the determination of the pH of a 0.010 M HNO2 solution, we will delve into the fascinating world of chemical equilibrium, weak acids, and the intricate interplay of factors that govern pH.
Our exploration will encompass the fundamental concepts of chemical equilibrium and its role in shaping pH, shedding light on the relationship between pH and hydrogen ion concentration. We will then delve into the properties of HNO2, examining its chemical formula, molecular weight, and acidity, while unraveling how its concentration influences the pH of a solution.
Chemical Equilibrium and pH

Chemical equilibrium is a state in which the forward and reverse reactions of a chemical reaction occur at the same rate. In this state, the concentrations of the reactants and products do not change over time. The position of equilibrium is determined by the relative strengths of the forward and reverse reactions.
The pH of a solution is a measure of its acidity or alkalinity. It is defined as the negative logarithm of the hydrogen ion concentration. The pH scale ranges from 0 to 14, with 0 being the most acidic and 14 being the most alkaline (also known as basic).
A pH of 7 is neutral.
Relationship between pH and Hydrogen Ion Concentration
The pH of a solution is directly related to the hydrogen ion concentration. The higher the hydrogen ion concentration, the lower the pH. The lower the hydrogen ion concentration, the higher the pH.
pH =
log[H+]
Where:
- pH is the pH of the solution
- [H+] is the hydrogen ion concentration in moles per liter
Properties of HNO2

Nitrous acid (HNO2) is a weak acid with the molecular weight of 47.01 g/mol. It is a colorless liquid that is soluble in water. The acidity of HNO2 is due to the presence of the hydrogen ion (H+). The concentration of H+ ions in a solution of HNO2 determines the pH of the solution.
Effect of HNO2 Concentration on pH
The concentration of HNO2 in a solution affects the pH of the solution. The higher the concentration of HNO2, the lower the pH of the solution. This is because the higher the concentration of HNO2, the more H+ ions are present in the solution.
The more H+ ions present, the lower the pH of the solution.
pH Calculations for Weak Acids
Determining the pH of a weak acid solution, such as HNO2, requires a specific approach. This involves understanding the dissociation constant (Ka) and its role in pH calculations.
Dissociation Constant (Ka)
The dissociation constant (Ka) is a quantitative measure of the strength of an acid. It represents the equilibrium constant for the dissociation reaction of the acid in water. A smaller Ka value indicates a weaker acid, while a larger Ka value indicates a stronger acid.
pH Calculations
To calculate the pH of a weak acid solution, follow these steps:
- Write the dissociation equation:HNO 2(aq) + H 2O(l) ⇌ H 3O +(aq) + NO 2–(aq)
- Set up the equilibrium expression:Ka = [H 3O +][NO 2–] / [HNO 2]
- Assume that x is the concentration of H3O +and NO 2–ions produced at equilibrium.
- Substitute x into the equilibrium expression:Ka = x 2/ ([HNO 2]
x)
- Solve for x:x = [H 3O +] = sqrt(Ka
[HNO2])
- Calculate the pH:pH =
log[H3O +]
Numerical Example

Let’s illustrate the calculation of pH for a 0.010 M HNO2 solution:
Ka Expression
The dissociation constant (Ka) for HNO2 is 4.5 x 10 -4.
ICE Table
We set up an ICE table to track the changes in concentrations:
| | HNO2 | H+ | NO2- ||:—|:—|:—|:—|| Initial | 0.010 | 0 | 0 || Change |
x | +x | +x |
| Equilibrium | (0.010
x) | x | x |
Substituting into Ka Expression
Substituting the equilibrium concentrations into the Ka expression:
“`Ka = [H+][NO2-] / [HNO2]
- 5 x 10 -4= x 2/ (0.010
- x)
“`
Approximation
Since HNO2 is a weak acid, the change in its concentration (x) is small compared to its initial concentration (0.010). Therefore, we can approximate (0.010 – x) as 0.010:
“`
5 x 10-4= x 2/ 0.010
“`
Solving for x, Find the ph of a 0.010mhno2 solution.
Solving for x, which represents the equilibrium concentration of H+:
“`x 2= 4.5 x 10 -6x = 6.7 x 10 -3“`
Calculating pH
Finally, we calculate the pH using the equilibrium concentration of H+:
“`pH =
log[H+]
pH =
log(6.7 x 10-3)
pH = 2.17“`
Factors Affecting pH: Find The Ph Of A 0.010mhno2 Solution.

The pH of a solution can be affected by several factors, including temperature and the presence of other ions. Temperature affects the equilibrium constant of the dissociation reaction, and thus the pH. Generally, as temperature increases, the equilibrium constant decreases, and the pH increases.
This is because the dissociation reaction is exothermic, and increasing the temperature shifts the equilibrium to the left, favoring the formation of the undissociated acid.
The presence of other ions can also affect the pH of a solution. Ions that have a common ion effect can decrease the dissociation of the weak acid, leading to a higher pH. For example, if we add sodium nitrite (NaNO 2) to a solution of nitrous acid (HNO 2), the sodium ions (Na +) will have a common ion effect with the hydrogen ions (H +) produced by the dissociation of nitrous acid, reducing the concentration of H +ions and increasing the pH.
Applications of pH

The pH of a solution plays a crucial role in various scientific disciplines, including chemistry, biology, and environmental science. Understanding the pH value is essential for numerous applications, ranging from industrial processes to medical diagnostics and environmental monitoring.
Chemistry
In chemistry, pH is a key factor in determining the reactivity of chemical substances. For instance, in acid-base reactions, the pH determines the extent of proton transfer and the formation of products. pH also affects the solubility and stability of chemical compounds, influencing their behavior in different chemical processes.
Biology
In biology, pH is critical for maintaining the proper functioning of living organisms. The pH of body fluids, such as blood and digestive juices, is tightly regulated to support essential biological processes. Deviations from optimal pH levels can disrupt enzyme activity, alter protein structure, and impair cell viability.
Environmental Science
In environmental science, pH is a crucial parameter for assessing water quality and ecosystem health. The pH of water bodies influences the solubility and bioavailability of nutrients and pollutants, affecting the distribution and abundance of aquatic organisms. Monitoring pH levels is essential for managing water resources, controlling pollution, and protecting aquatic ecosystems.
General Inquiries
What is pH?
pH is a measure of the acidity or alkalinity of a solution, ranging from 0 to 14. A pH of 7 is neutral, while values below 7 indicate acidity and values above 7 indicate alkalinity.
What is the relationship between pH and hydrogen ion concentration?
pH is inversely proportional to hydrogen ion concentration. As hydrogen ion concentration increases, pH decreases, and vice versa.
How does the concentration of HNO2 affect the pH of a solution?
As the concentration of HNO2 increases, the pH of the solution decreases. This is because HNO2 is a weak acid that dissociates in water to produce hydrogen ions, which lower the pH.