Chemistry i worksheet classification of matter and changes – Delve into the fascinating world of chemistry with our comprehensive worksheet on matter classification and changes. This guide unlocks the secrets of the material world, providing a solid foundation for understanding the fundamental principles that govern the interactions of substances.
Embark on a journey through the diverse states of matter, unraveling their unique properties and characteristics. Witness the transformative power of physical and chemical changes, and gain insights into the factors that influence their occurrence.
Introduction to Chemistry
Chemistry is the study of matter and its properties, composition, and changes. It plays a vital role in understanding the world around us, from the air we breathe to the food we eat. Chemistry is divided into different branches, including organic chemistry, inorganic chemistry, physical chemistry, and analytical chemistry, each with its own focus and applications.
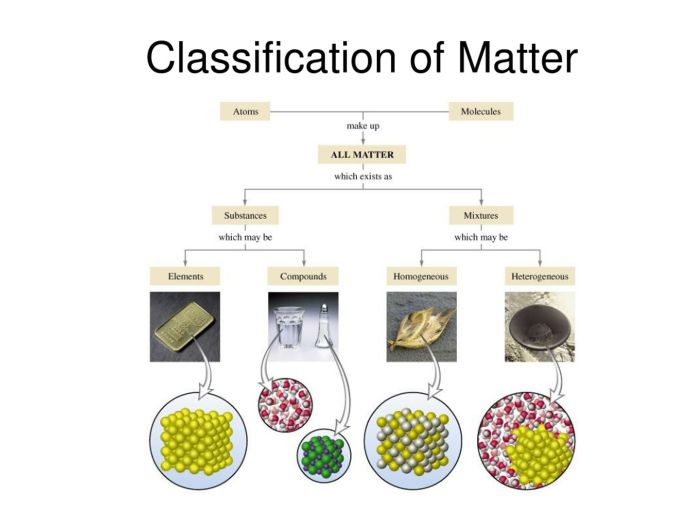
Classification of Matter: Chemistry I Worksheet Classification Of Matter And Changes
States of Matter
Matter exists in three primary states: solid, liquid, and gas. Solids have a definite shape and volume, liquids have a definite volume but no definite shape, and gases have neither a definite shape nor volume. The properties and characteristics of each state of matter are determined by the arrangement and motion of its particles.
Types of Matter
Matter can be classified into two main types: elements and compounds. Elements are pure substances that cannot be broken down into simpler substances by chemical means. Compounds are substances composed of two or more elements chemically combined in fixed proportions.
Physical and Chemical Changes
Physical Changes
Physical changes are changes in the form or appearance of a substance without altering its chemical composition. Examples include melting, freezing, sublimation, and boiling.
Chemical Changes
Chemical changes involve the rearrangement of atoms to form new substances. These changes are irreversible and result in the formation of new substances with different properties. Examples include burning, rusting, and digestion.
Factors Affecting Chemical Reaction Rates, Chemistry i worksheet classification of matter and changes
The rate of chemical reactions can be affected by factors such as temperature, concentration, surface area, and the presence of a catalyst.
Mixtures and Compounds
Mixtures
Mixtures are combinations of two or more substances that are not chemically bonded. They can be separated by physical means, such as filtration or distillation.
Compounds
Compounds are substances composed of two or more elements that are chemically combined in fixed proportions. They cannot be separated by physical means and have unique properties that differ from their constituent elements.
Methods for Separating Mixtures
Methods for separating mixtures include filtration, distillation, chromatography, and crystallization.
Elements and the Periodic Table
Elements
Elements are the fundamental building blocks of matter and cannot be broken down into simpler substances by chemical means. They are arranged in the periodic table, which organizes elements based on their atomic number, electron configuration, and chemical properties.
Periodic Table Organization
The periodic table is organized into groups (vertical columns) and periods (horizontal rows). Elements in the same group have similar chemical properties, while elements in the same period have the same number of electron shells.
Trends in Chemical Properties
There are trends in chemical properties across the periodic table. For example, elements in the same group tend to have similar ionization energies and electronegativities.
FAQs
What is the difference between a physical change and a chemical change?
Physical changes involve alterations in the physical properties of a substance, such as shape, size, or state, without altering its chemical composition. Chemical changes, on the other hand, result in the formation of new substances with different chemical properties.
How can I classify different types of matter?
Matter can be classified into three main states: solid, liquid, and gas. Solids have a definite shape and volume, liquids have a definite volume but no definite shape, and gases have neither a definite shape nor volume.
What is the significance of the periodic table?
The periodic table organizes elements based on their atomic number, electron configuration, and chemical properties. It provides a systematic framework for understanding the behavior and interactions of elements.
