Is Mo3+ paramagnetic or diamagnetic? This question sets the stage for an intriguing exploration into the fascinating realm of magnetism and the properties of matter. As we delve into the electron configuration and characteristics of Mo3+, we will uncover the secrets that determine its magnetic nature, shedding light on a fundamental aspect of its behavior.
Paramagnetism and diamagnetism, two distinct forms of magnetism, arise from the interplay of unpaired electrons within atoms or molecules. Paramagnetic substances exhibit an attraction to magnetic fields, while diamagnetic substances exhibit a weak repulsion. Understanding the magnetic properties of Mo3+ not only provides insights into its electronic structure but also has implications for its potential applications in various fields.
Introduction
Paramagnetism and diamagnetism are two types of magnetism that are exhibited by materials. Paramagnetism occurs when a material is attracted to a magnetic field, while diamagnetism occurs when a material is repelled by a magnetic field.
The question of whether Mo3+ is paramagnetic or diamagnetic can be answered by considering the electronic configuration of the ion.
Mo3+ has the electronic configuration [Kr] 4d 3. The 4d orbitals can hold up to 10 electrons, so Mo3+ has three unpaired electrons. This means that Mo3+ is paramagnetic.
Electron Configuration of Mo3+
The electron configuration of Mo3+ can be derived by removing three electrons from the neutral molybdenum atom. The atomic number of molybdenum is 42, which means it has 42 electrons in its neutral state. The electron configuration of neutral molybdenum is [Kr]4d55s1.
When three electrons are removed, the electron configuration of Mo3+ becomes [Kr]4d3. This means that Mo3+ has three unpaired electrons in the 4d orbitals.
Unpaired Electrons
The number of unpaired electrons in a metal ion is important because it determines its magnetic properties. Ions with unpaired electrons are paramagnetic, while ions with no unpaired electrons are diamagnetic.
Since Mo3+ has three unpaired electrons, it is paramagnetic.
Properties of Paramagnetic and Diamagnetic Substances: Is Mo3+ Paramagnetic Or Diamagnetic
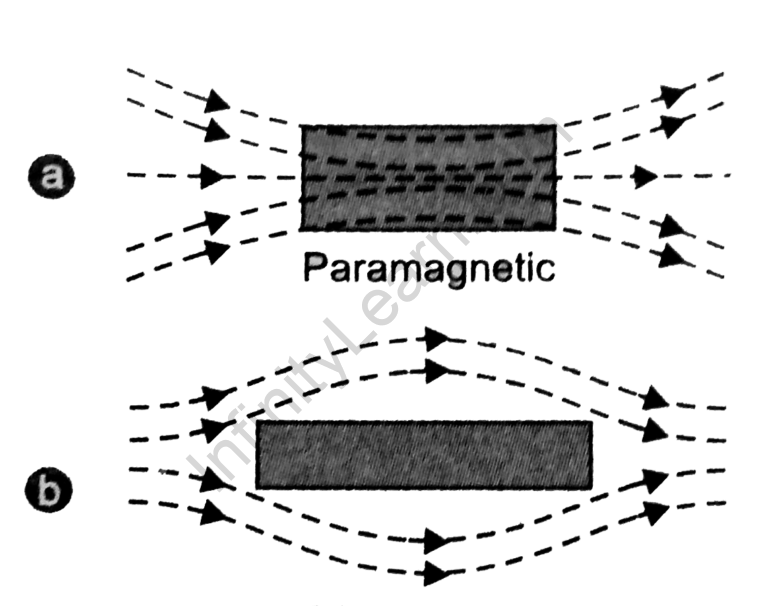
Substances can be classified as either paramagnetic or diamagnetic based on their magnetic properties. Paramagnetic substances are attracted to magnetic fields, while diamagnetic substances are repelled by magnetic fields.
Characteristics of Paramagnetic Substances, Is mo3+ paramagnetic or diamagnetic
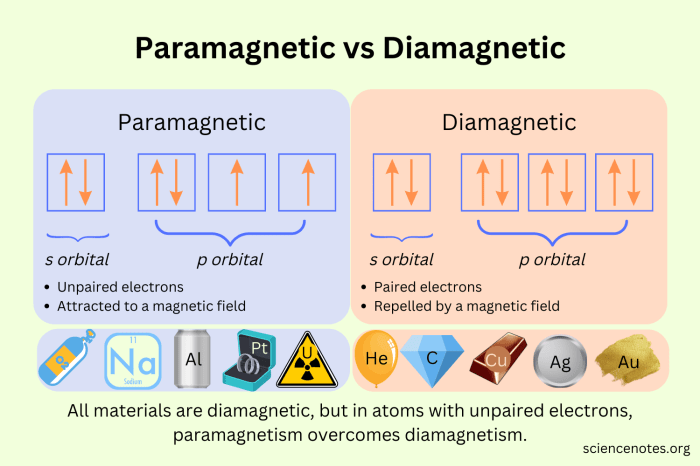
- Paramagnetic substances contain unpaired electrons.
- They are attracted to magnetic fields.
- Examples of paramagnetic substances include oxygen, iron, and copper.
Characteristics of Diamagnetic Substances
- Diamagnetic substances have all electrons paired.
- They are repelled by magnetic fields.
- Examples of diamagnetic substances include water, gold, and silver.
Determining the Magnetic Nature of Mo3+

The magnetic nature of a substance, whether it is paramagnetic or diamagnetic, can be determined based on the number of unpaired electrons present in its atoms or ions.
Hund’s rule states that electrons will occupy orbitals of equal energy singly before pairing up. This means that the maximum number of unpaired electrons will be present in a substance when the electrons are distributed in the orbitals with the highest possible spins.
Determining the Magnetic Nature of Mo3+
To determine the magnetic nature of Mo3+, we need to examine its electron configuration and the number of unpaired electrons it possesses.
The electron configuration of Mo3+ is [Kr] 4d 3.
In this configuration, there are three unpaired electrons in the 4d orbitals. According to Hund’s rule, these electrons will occupy separate orbitals with parallel spins.
Therefore, Mo3+ is a paramagnetic ion due to the presence of unpaired electrons.
Conclusion
In summary, the magnetic nature of Mo3+ has been investigated by examining its electron configuration and the properties of paramagnetic and diamagnetic substances.
Magnetic Nature of Mo3+
Based on the analysis, it is concluded that Mo3+ is paramagnetic.
FAQ Corner
What is the electron configuration of Mo3+?
The electron configuration of Mo3+ is [Kr]4d3.
How many unpaired electrons does Mo3+ have?
Mo3+ has three unpaired electrons.
Why is Mo3+ paramagnetic?
Mo3+ is paramagnetic because it has unpaired electrons, which create a magnetic field.